


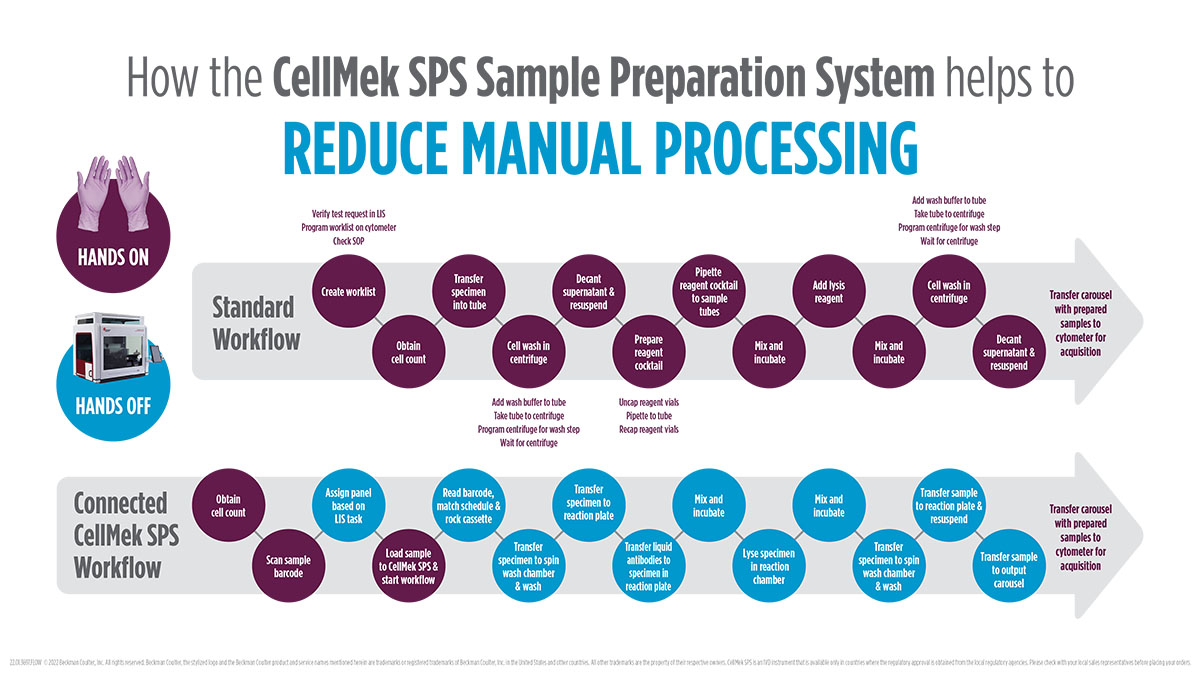
The CellMek SPS system helps you unlock the power of lean by addressing major process wastes related to sample preparation in your clinical flow cytometry laboratory.
- Drives Efficiency via Automation.
- Standardizes Processes & Reduces Potential for Error.
- Ensures Flexibility Via User-Defined Protocols.
- Provides Confidence in Results facilitated by a full audit trail.
- Frees up lab staff to deal with more value-added tasks.
Unlock the Power of Lean
The CellMek SPS System is designed to enable lean processing. The LEAN concept provides a systematic approach to look at workflows to take out all non-value adding process steps, the so-called process wastes, in order to increase efficiency. The CellMek SPS System addresses the challenges associated with complex sample preparation in flow cytometry to increase lab efficiency and helps you to cope with increasing workloads. Are you ready to Unlock the Power of Lean?

CellMek SPS Features
The CellMek SPS system helps your lab reach the next level of process optimization. It is designed to simultaneously manage various preparation methods in random access while providing continuous loading, optimizing efficiency and traceability.
Customized Flexibility
- Panel Designer Software lets you easily design your own protocols
- CellMek SPS takes care of scheduling - no need to batch by application
- Many options such as lysis, washing steps and volumes to pipet
Uninterrupted Workflow
- Onboard washing capability with customizable washing steps
- Both pre- and post-washes are optional steps of the sample prep workflow
- Automated rocking and cap piercing - no need to manually unscrew specimen tube caps
Optimized Time to Result
- Continuous loading & unloading of samples through tube-specific cassettes
- Parallel Processing to help optimize scheduling and sample throughput
- Output carousel can be retrieved to start sample analysis while system prepares more samples
Traceable Inventory
- A refrigerated 53-position liquid antibody carousel means reagents can remain onboard
- Capacity for up to 12 DURACartridges - our innovative new format for dry reagents
- All reagents and consumables are barcoded to provide automated tracking for a full audit trail

CellMek SPS: Audit Trail Features

Table 1. Information available in the Audit Trail
| Category | Information |
|---|---|
| Panels & Worklist | |
| Panel added/removed to/from specimen | Panel Name, Specimen Barcode, LIS Order (Yes / No) |
| Worklist created | Worklist file successfully generated to (Network path / path on USB drive) |
| Consumables | |
| Liquid reagent vial | Reagent Name, Part Number, Regulatory status, Lot, Exp. Date, Remaining Volume |
| DURACartridge (Dry reagents) | Reagent Name, Part Number, Lot, Date of Manufacture, Exp. Date, Number of Tests, Number of Remaining Tests |
| Diluent reagent | Reagent Name, Part Number, Regulatory status, Lot, Exp. Date, Remaining Volume |
| External Resource | |
| Status on Condensate, Diluent, Waste | full / empty |
| Instrument Monitoring & Access | |
| Status on Liquid Antibody Module | opened / closed / open > 5 min |
| Liquid Antibody Module temperature is outside the range of 2°C to 8°C | Temperature in °C |
| Status on output carousel | Drawer opened / closed |
| Status on reaction plate module | Lid closed /opened |
| Maintenance / Service | |
| Shutdown | Instrument shutdown completed successfully |
| Maintenance Prime Clean Cell Wash | Prime and Clean Cell Wash procedure completed successfully / failed |
Click on the sections below and learn more about the intuitive software interface to retrieve the audit trail, sample and specimen reports.
The CellMek SPS is an automated sample preparation system intended for in vitro diagnostic use that can be programmed by the user to perform a variety of liquid handing operations, including sample preparation for flow cytometry. It is designed to automate staining, lysing, incubating, and washing of different biological specimen types, which enhances productivity by minimizing resource allocation for repetitive work in the clinical laboratory. The purpose of this study is to demonstrate the precision performance of CellMek SPS (repeatability and reproducibility) using a representative sample preparation workflow that utilizes the Cell Wash Module (CWM) as well as the Dry Reagent Module, which supports the DURACartridge custom dry reagent format.
Table 1. DURACartridge Custom Dry 10C Panel.
| DURACartridge 10C Panel | 488 nm | 633 nm | 405 nm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FITC | PE | ECD | PC5.5 | PC7 | APC | APC-A700 | APC-A750 | PB | Krome Orange | |
| Markers | Kappa | Lambda | CD10 | CD5 | CD200 | CD34 | CD38 | CD20 | CD19 | CD45 |
Methods
The test cases in this study utilized a Wash-Stain-Lyse/Fix-Wash workflow with a 10-Color (10C) cocktail in DURACartridge dry format and the IOTest 3 +0.25% fixative lysing solution. The 10C cocktail included Kappa-FITC, Lambda-PE, CD10-ECD, CD5-PC5.5, CD200-PC7, CD34-APC, CD38-AA700, CD20-AA750, CD19-PB, and CD45-KrO. Data from samples prepared by the CellMek SPS were acquired on a Navios flow cytometer and analyzed using Kaluza C software. Standard Deviation (SD) and Coefficient of Variations (%CV) were calculated for each marker by each instrument and across all instruments.
For repeatability testing, peripheral blood specimens obtained from normal donors were spiked with CD34+ KG1a Cells and processed by three CellMek SPS instruments (1 donor/instrument, 10 replicates/donor). A single-output-tube panel was defined to process 100 μL of specimen at a time, which was then run in replicate thus eliminating any system variability due to multi-dispense of specimen.
Analysis Strategy for Repeatability:
- The data files of the repeatability samples were analyzed offline using Kaluza C v1.1 software.
- Standard deviation (SD) and coefficient of variations (%CV) were calculated for each marker by each instrument (Table 3).
- Repeatability variability was compared to the specified repeatability acceptance criteria (Table 2).
For reproducibility testing, one lot of ClearLLab Control Cells, Abnormal (part number B90003) was processed on three CellMek SPS instruments. To ensure even use of both assemblies within the CWM and capture of maximum module variability, a two-output-tube panel was defined to process 100 μL of specimen in duplicate per specimen tube run, which was then assigned to two identical specimen tubes that were run in parallel (4 output tubes total). Specimen pairs were run in duplicate (8 output tubes) at least once a day (AM and/or PM) for at least five days for a total of 80 replicates per instrument. The AM and PM runs were processed at least 2 hours apart.
Analysis Strategy for Reproducibility:
- The data files were analyzed offline using Kaluza C v1.1 software.
- Standard deviation (SD) and coefficient of variations (%CV) were calculated for each marker by each instrument and across all instruments (Tables 4 and 5).
- Variability was compared to the specified reproducibility acceptance criteria (Table 2).
- ClearLLab Control Cells Abnormal were used and assessed for % positive cell populations measured against the lot-specific assay sheet (Table 6)
Table 2. Precision Acceptance Criteria.
| Parameter | Repeatability | Reproducibility |
|---|---|---|
| ≤ 20% Gated | SD < 2 | SD < 2 |
| > 20% Gated | < 10% CV | < 10% CV |
DURACartridges
Our innovative new format for automated use of dry reagents
DURACartridges are a new format of custom designed dry pre-mixed antibody panels to work with the CellMek SPS Sample Preparation System. Our proprietary method to dry reagents with the DURA Innovations technology does not involve the process of lyophilization and offers many advantages for improved workflow efficiency. Every DURACartridge has 12 wells with dry reagents covered with pierceable foil. The CellMek SPS System provides onboard capacity of up to 12 DURACartridges, 12 tests each, which adds up to a total capacity of up to 144 tests. That can help further decrease the turnaround time in labs with higher throughput.


DURACartridges can be ordered through Custom Reagent Services. This service offers flexibility to design a reagent and panel configuration, using Beckman Coulter’s large antibody portfolio, as well as choose the preferred reagent format in order to simplify workflow and minimize time spent on manual processes. Depending on their needs, laboratories can choose either LUCID Custom Panel Design and Cocktail Services* or RESOURCE Contract Manufacturing Services**
Find out more about the differences between LUCID and RESOURCE services.
 Forgalmazott termékeink gyártói - keressen gyártó szerint a logóra kattintva
Forgalmazott termékeink gyártói - keressen gyártó szerint a logóra kattintva





